Nature | Fan Rong and Tang Li Teams Publish Groundbreaking Studies on Cancer Immunotherapy
In recent years, Type-1 immunity has been recognized as the primary immune response for combating tumors. However, Type-2 immunity has garnered increasing attention due to its more complex role in tumor biology. While often associated with an immunosuppressive microenvironment that promotes tumor growth, emerging research suggests that Type-2 immunity may also help inhibit tumor progression. Despite this, clinical studies exploring the relationship between Type-2 immunity and cancer treatment remain limited.
On September 25, 2024, two groundbreaking studies were published in Nature by Professor Fan Rong from Yale University and Professor Tang Li from the Swiss Federal Institute of Technology in Lausanne (EPFL). The papers, titled Single-cell CAR T atlas reveals type 2 function in 8-year leukemia remission and The type 2 cytokine Fc–IL-4 revitalizes exhausted CD8+ T cells against cancer, offer new insights into the role of Type-2 immunity in cancer therapy.
Dr. Zhiliang Bai from Yale University and Dr. Bing Feng from EPFL are the co-first authors of these pioneering studies.
First Study: Type-2 Immunity's Role in Long-Term CAR-T Therapy Success
The first study utilized single-cell multi-omics sequencing to investigate pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL) who underwent CD19 CAR-T therapy. The researchers found that Type-2 CAR-T cells were significantly more prevalent in patients who achieved long-term, 8-year remission compared to those with shorter remission periods. This discovery challenges the traditional view that Type-2 immunity is detrimental to cancer therapy, especially in hematological cancers. Instead, the study highlights the crucial role of Type-2 immunity in enhancing the durability of CAR-T treatments.
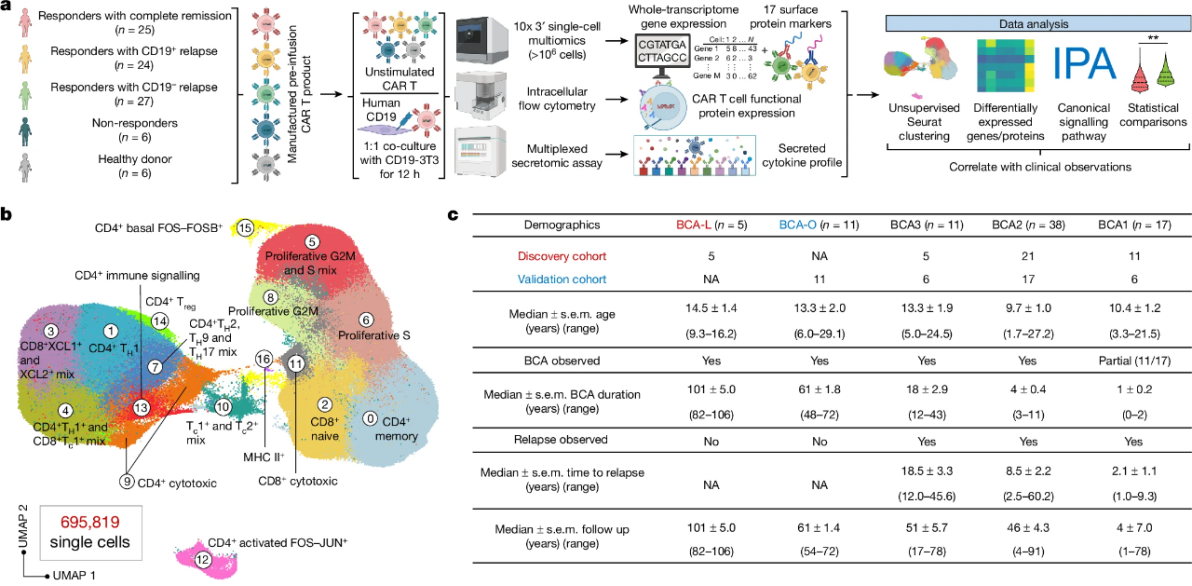
Second Study: Fc-IL-4 Cytokine Enhances Solid Tumor Clearance
The second study focused on the development and application of a novel Type-2 cytokine, Fc-IL-4. When used in combination with existing CAR-T therapies and immune checkpoint inhibitors, Fc-IL-4 significantly boosted the elimination of solid tumors. In experimental models of melanoma and colon cancer, Fc-IL-4 worked synergistically with Type-1 immunotherapy to produce a durable anti-tumor immune response. This research not only demonstrates the potential of Type-2 immune factors in enhancing cancer treatment but also reveals that Type-1 and Type-2 immunity can cooperate, rather than oppose each other, to create a more effective immunotherapy response.
Cell | Fan Rong's Team Develops the World's First Clinical-Grade FFPE Tissue Spatial Whole Transcriptome Sequencing Technology
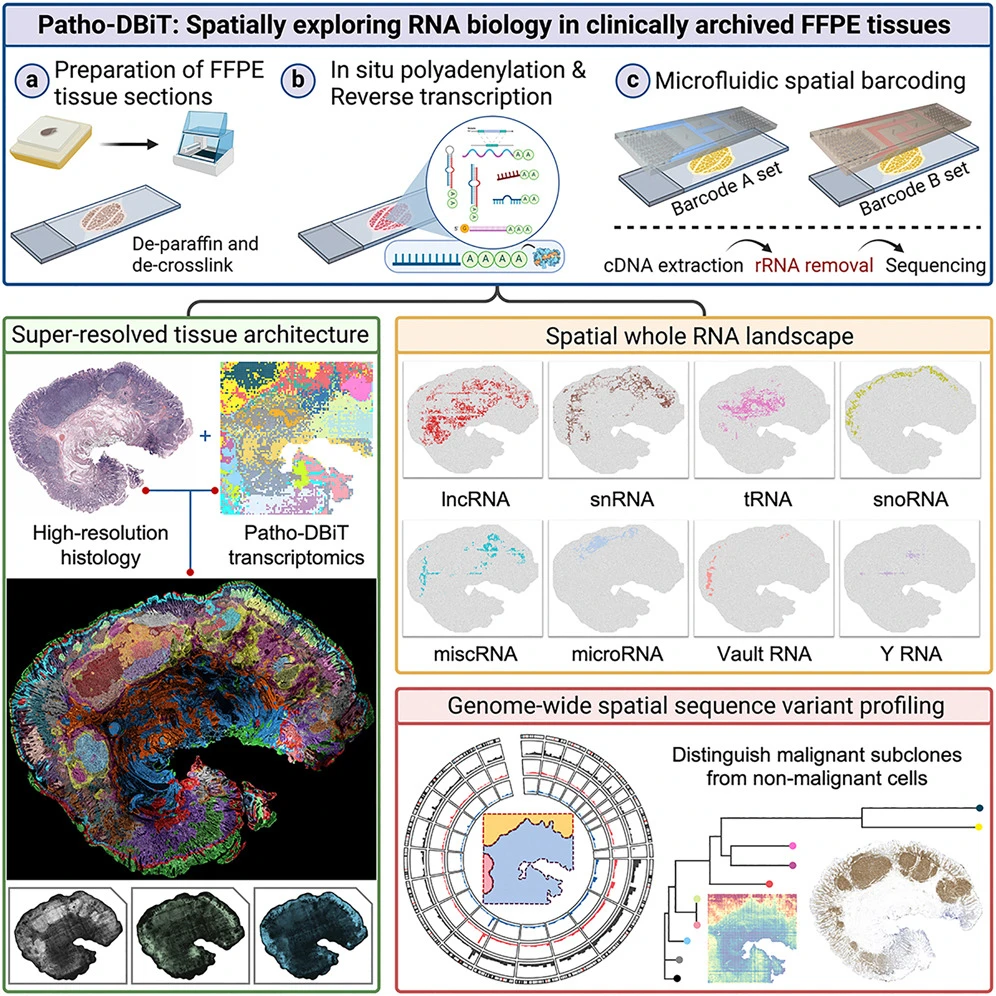
The ability to spatially explore RNA biology in formalin-fixed, paraffin-embedded (FFPE) tissues has revolutionized the field of tissue pathology. On September 30, 2024, Fan Rong’s team at Yale University made a significant advance in this domain by publishing a study in Cell titled Spatially exploring RNA biology in archival formalin-fixed paraffin-embedded tissues.
Dr. Zhiliang Bai and colleagues are the lead authors of this landmark paper.
Patho-DBiT: A Revolutionary Tool for Clinical FFPE RNA Analysis
The research team introduced a new computational method, Patho-DBiT (Pathology-Compatible Tissue-Determined Barcode for In Situ Polyadenylation), designed specifically for analyzing archived FFPE samples. This innovative technique allows for the spatial co-analysis of gene expression and RNA processing, providing unprecedented insights into region-specific splicing isoforms and high-sensitivity transcriptomic profiles of tumor tissues stored for over five years.
Patho-DBiT can also capture genome-wide single-nucleotide RNA variants, making it possible to distinguish malignant subclones from non-malignant cells in human lymphomas. Furthermore, Patho-DBiT maps microRNA regulatory networks and RNA splicing dynamics, helping researchers decode the roles of these factors in spatial tumorigenesis.
At the single-cell level, Patho-DBiT dissects the spatiotemporal cellular dynamics that drive tumor clonal evolution and progression. This technique is poised to become an invaluable tool for uncovering the rich RNA biology in FFPE tissues and advancing clinical pathology assessments.


 (+86)18613012387
(+86)18613012387 info@royallee.cn
info@royallee.cn EN
EN CN
CN TH
TH IDN
IDN  AR
AR


