The 2024 ESMO Asia Congress, held from December 6–8 in Singapore, served as a premier platform for groundbreaking discussions in oncology across the Asia-Pacific region. The event unveiled numerous global and regional advancements in cancer research and clinical practice.
Among the highlights was a presentation by Professor Lu Shun from Shanghai Chest Hospital, Shanghai Jiao Tong University School of Medicine. He reported on a Phase III study (POD1UM-304) assessing the efficacy of Retifanlimab combined with platinum-based chemotherapy as a first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC), encompassing both squamous and non-squamous histologies.
Study Background
The prognosis for advanced NSCLC remains dismal, underscoring the urgent need for innovative therapeutic strategies. Clinical evidence suggests that combining immune checkpoint inhibitors (CPIs) with standard-of-care (SOC) chemotherapy can deliver survival benefits in advanced NSCLC. The POD1UM-304 study aimed to evaluate the efficacy and safety of Retifanlimab, a PD-1 inhibitor, in combination with platinum-based chemotherapy for this patient population.
Study Design and Methodology
The study enrolled patients aged 18 years or older with metastatic NSCLC who had not received prior systemic treatment for advanced disease. Eligible participants were free of EGFR, ALK, BRAF, or ROS1 mutations or rearrangements. Patients were randomized in a 2:1 ratio to receive either Retifanlimab (375 mg) or a placebo, in combination with SOC platinum-based chemotherapy.
Treatment regimens included:
- Non-squamous NSCLC: Pemetrexed with cisplatin/carboplatin every 3 weeks for 4 cycles, followed by maintenance therapy with pemetrexed every 3 weeks.
- Squamous NSCLC: Carboplatin with paclitaxel or nab-paclitaxel every 3 weeks for 4 cycles.
Patients were treated for up to 2 years, with overall survival (OS) as the primary endpoint. Secondary endpoints included:
- Progression-free survival (PFS),
- Objective response rate (ORR),
- Duration of response (DOR),
- Safety, and
- Pharmacokinetics.
Study Results
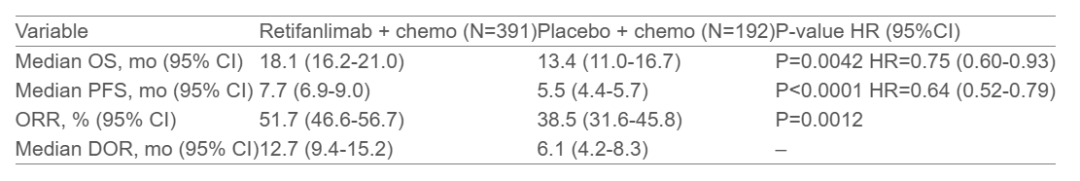
A total of 583 patients were randomized, including 381 with non-squamous NSCLC and 202 with squamous NSCLC, with a median age of 64 years (range: 29–84 years). The study met all endpoints, demonstrating significant improvement in outcomes for the Retifanlimab plus chemotherapy group compared to the placebo plus chemotherapy group. The detailed results are summarized below:
Safety Profile
In the Retifanlimab group, there was a higher incidence of severe and grade ≥3 treatment-emergent adverse events (TEAEs), as well as TEAEs leading to treatment interruption or discontinuation. This trend correlated with the longer treatment exposure in this group. Notably, the addition of Retifanlimab did not result in an increase in fatal or severe TEAEs related to COVID-19.
Study Conclusion
Adding Retifanlimab to platinum-based chemotherapy improved overall survival (OS), with a safety profile consistent with other checkpoint inhibitors. Integrating Retifanlimab into SOC first-line chemotherapy shows promise as a novel treatment option for metastatic NSCLC.


 (+86)18613012387
(+86)18613012387 info@royallee.cn
info@royallee.cn EN
EN CN
CN TH
TH IDN
IDN  AR
AR




