Iodine-131 treatment
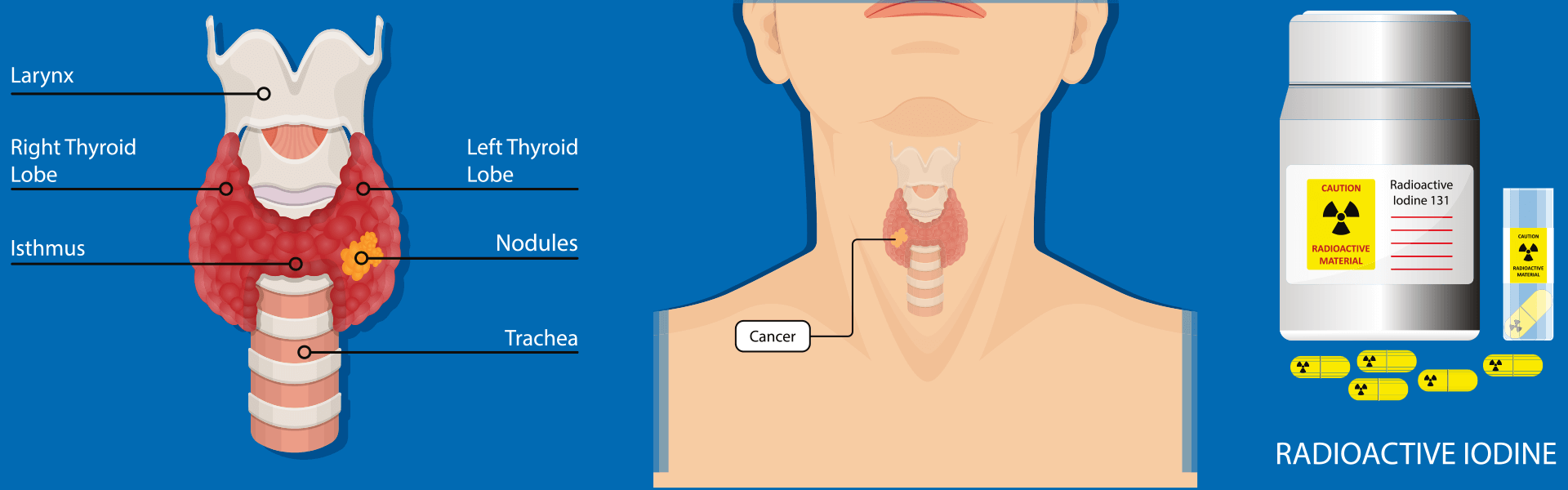
The method of Iodine-131 treatment involves the oral administration of a Iodine-131 solution or capsules, entering the bloodstream through the digestive system. It selectively targets and accumulates in residual thyroid cells and residual thyroid tumor cells. By emitting β-rays through decay, it induces swelling, degeneration, and necrosis in the targeted cells, effectively eliminating residual thyroid tissue and cancerous lesions. The objective is to reduce tumor recurrence and metastasis. The average range of β-rays within tissues is less than 1mm, with almost all energy released within residual thyroid tissue or metastatic lesions, resulting in minimal impact on surrounding normal tissues and organs.
| Clinical Applications | Solution | Radioactive Iodine-131 in solution tends to adhere to the patient's oral cavity, nasal cavity, esophagus, etc., potentially causing taste abnormalities, mucosal inflammation, swelling, and pain. |
| Capsule | Administered in capsule form, allowing the medication to reach the stomach directly, thus avoiding these adverse reactions. | |
| Operational Usage | Solution | Before clinical use, the solution typically requires appropriate dilution. Depending on the patient's condition, healthcare professionals extract varying volumes of the solution for oral administration. |
| Capsule | Capsule form eliminates the process of healthcare professionals dispensing oral solution, reducing unnecessary radiation exposure for both healthcare professionals and patients. |
Iodine-131, with a half-life of 8.02 days, has a variety of applications. They include diagnostics and therapy of thyroid gland (either in the form of solution or capsule), industrial tracers and various research applications such as antibody labelling. Iodine-131 is also used to label antibodies for therapeutic applications in cancer treatment.
Relevant Articles:
-Well-differentiated Thyroid Cancer & Iodine 131 Therapy-

-Thyroid Health Awareness-
-Thyroid Cancer Warning Signs-
